Vinyl sulfonimidamides as a new cysteine reactive electrophile
August 19th, 2025 By John Widen
One way into my heart is through covalent small molecules.
I wrote a previous article
about Nura Bio developing acrylamide-containing covalent inhibitors
targeting Cys311 near an allosteric binding pocket on SARM1. Acrylamides are highly represented
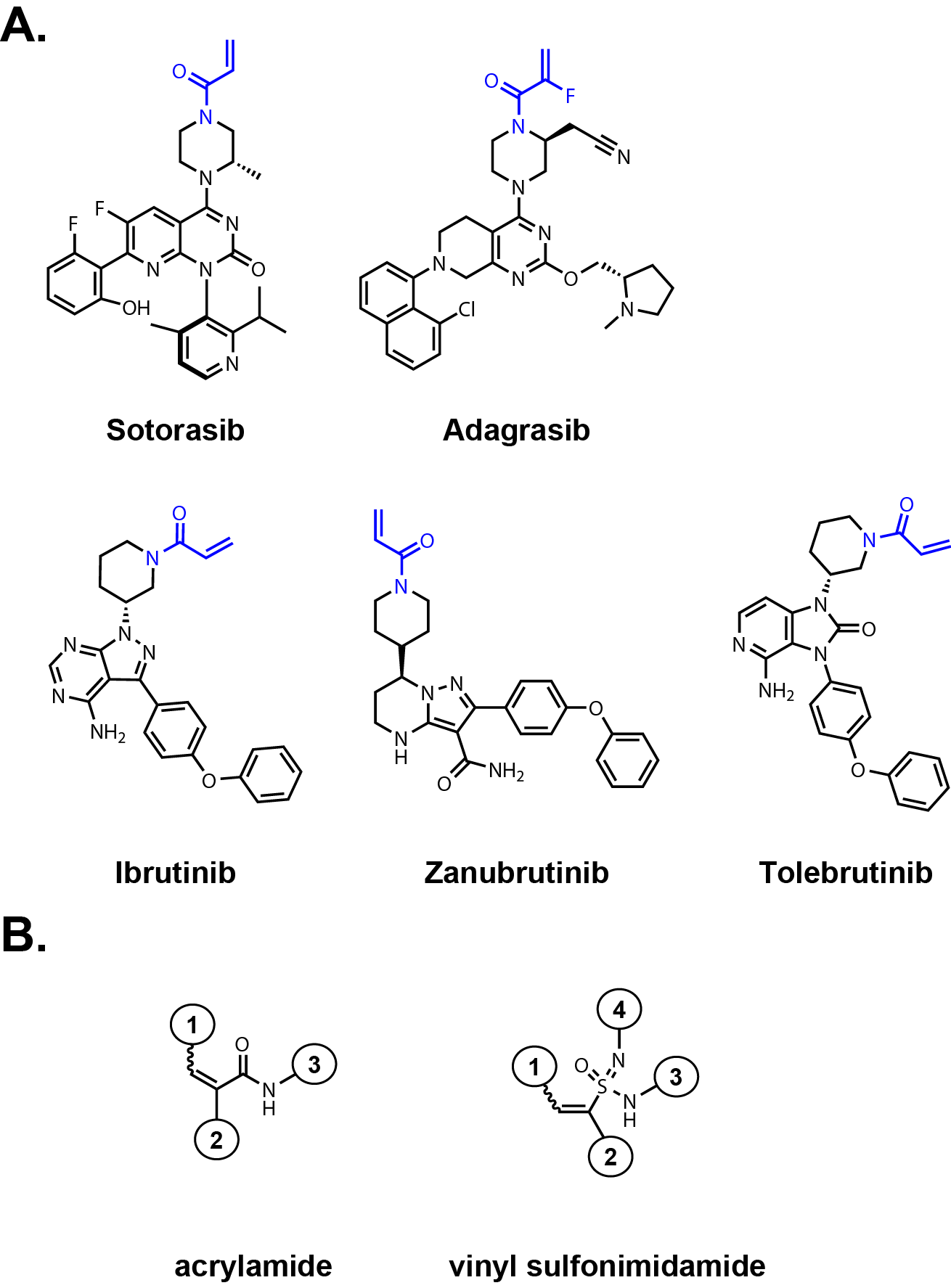
in recent FDA-approved drugs with covalent mechanism of actions including KRASG12C inhibitors Sotorasib
and Adagrasib (Fig. 1A). FDA approved covalent BTK (Bruton’s tyrosine kinase) inhibitors including
Ibrutinib and Zanubrutinib also contain acrylamides to react with Cys481 within the active site.
Another brain penetrant covalent inhibitor of BTK, Tolebrutinib, will likely be approved
by the FDA late 2025, early 2026 after a
favorable phase 3 clinical trial for treatment of
non-relapsing secondary progressive multiple sclerosis. This would serve as an example of an
FDA approved, chronically administered, brain penetrant covalent small molecule.
Acrylamide reactivity can be tuned through substitutions at the alpha and beta positions
of the olefin as well as modulated through the amine attachment point (Fig. 1B). One common modification
is an alpha-fluorine substitution to attenuate acrylamide reactivity, which was key to the
discovery of Adagrasib.
It is counter intuitive to have an electron withdrawing halogen at the alpha position reduce
reactivity of an acrylamide, but I believe the explanation is that fluorine removes electron density
from the alpha carbon and reduces conjugation with the carbonyl group, thereby reducing the
electrophilicity of the beta carbon. Beta-methyl acrylamide substitutions significantly reduce
thiol reactivity but other beta substitutions such as NMe2-methylene or aryl
substitutions can have various effects. Alpha-cyano acrylamides are reversible covalent towards
thiols because of the acidity of the alpha carbon after hetero-Michael addition.
The amide substitutions can modulate the electrophilicity of an acrylamide as well.
Trends of general reactivity are more predictive where electron withdrawing substitutions typically increase
the reactivity of the acrylamide whereas electron donating groups reduce the reactivity.
These effects are more challenging to predict in the context of reactivity with a nucleophilic
amino acid on the protein target of interest when substitutions can also have effects on the binding
component of the molecule-protein interaction prior to covalent reaction.
The three locations to modulate the reactivity of an acrylamide
leaves quite a few combinations given the functional group possibilities.
Imagine all of the possible substitutions that have and can be introduced at these three positions to make
many combinations. However, not all modifications at every position are tolerated within a binding pocket.
The rest of the molecule extending from the amide nitrogen is (hopefully) driving a specific binding
event and there will be limitations on the substitutions that can be made to tune the reactivity of the acrylamide.
There is always room for improvement!
This is where a
recent publication
comes in to expand the possibilities available
to tune an electrophile’s reactivity. This new publication takes advantage of the imide of
vinyl sulfonimidamides to introduce substituents that affect the electrophile’s reactivity toward thiols (Fig. 1B).
Sulfonimidamides are becoming another functional group in a medicinal chemist’s toolbox to fine tune
properties of small molecules. Sulfones and sulfonamides are consistently incorporated into bioactive
molecules during optimization. Sulfones are versatile in a way that provides polarity to a molecule
but at the same time tolerated in hydrophobic areas of a pocket and resistant to metabolism.
Sulfones also provide a unique geometry as a linker compared to carbon, nitrogen and oxygen.
The sulfone and sulfonamide brethren, sulfonimidamide, provides another sulfur based functional group;
unique because of the imide nitrogen that introduces a chiral center at the sulfur and provides
an additional vector for substitutions. Synthetic accessibility of sulfonimidamides is improving as well.
Incorporating this functional group into molecules may be more feasible with
recent one-pot synthetic methods.
Although, the additional chiral center is not ideal, I’m sure this unique functional group
will have specific use cases where there is an opportunity to grow into a
vector that would otherwise not be accessible or to make a key interaction.
Getting back to the aforementioned publication by Michael Willis’ group at the
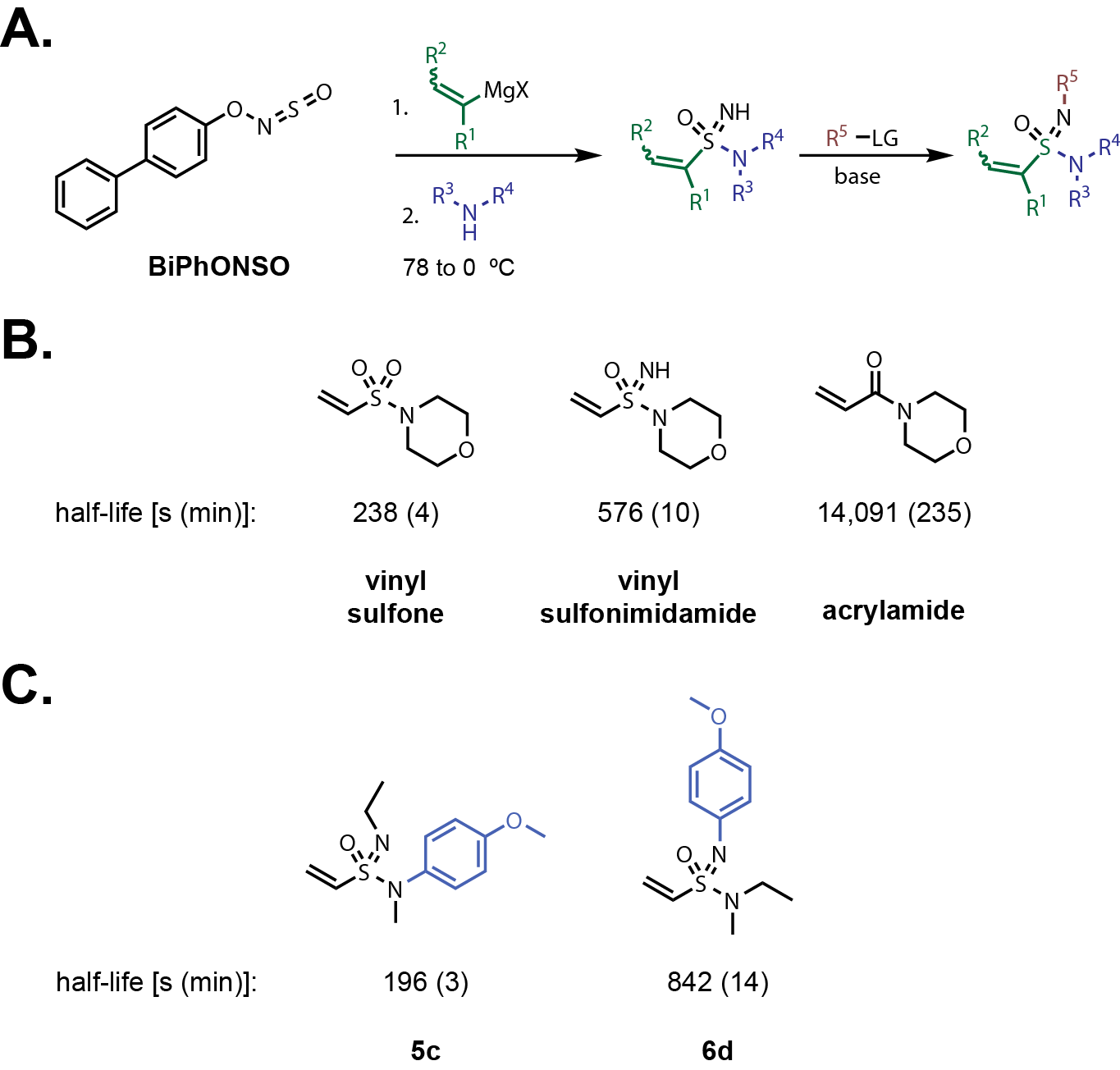
University of Oxford, they demonstrate a straight-forward synthesis of vinyl sulfonimidamides using a reagent
developed by the same laboratory (Fig. 2A).
Previous methods required reagents that are challenging to handle and an oxidation step from Sulfur(IV)
to Sulfur(VI). This new N-sulfinylhydroxylamine
reagent, BiPhONSO, can be purchased from what appears to be a single vendor, Cortex Organics.
It looks like this small chemical vendor was founded by a couple of colleagues at Oxford and
teamed up with Michael Willis’ lab to sell the BiPhONSO reagent. The reagent can also be synthesized
in one step from commercially available materials, O-biphenyl hydroxyl amine and SOCl2.
BiPhONSO is a solid at room temperature and seems to balance reactivity, ease of use, and safety.
(Side note: I'm pronouncing BiPhONSO as (Bye-Fonso). Not sure if that is intentional but I think it is catchy!)
Using a one-pot procedure developed by the Willis lab, vinyl sulfonimidamides can be made from
sequential addition of a vinyl Grignard and an amine to BiPhONSO. The corresponding imide can be
substituted with various electrophiles in the presence of base. The imide substitution is the variable
piece that the authors use to tune the reactivity of the electrophile. The reactions proceed
in minutes after warming from -78 to 0 Cº. The syntheses seem very straight forward and quick, which is nice!
The authors used this procedure to produce a small library and evaluate reactivities of
matched pairs with glutathione (GSH) under physiological conditions (pH 7.3). Second-order half-life is reported
for the majority of substrates because the kinetics were too fast to accurately measure under pseudo-first order conditions.
They focused on morpholino and 4-methoxyanliline
amines with varying imine substitutions for comparison. The unsubstituted sulfonimidamide was between
the reactivity of the corresponding sulfonamide and acrylamide (Fig. 2B). However, the half-life is much
closer to the vinyl sulfonamide
(Half-lives: vinyl sulfonamide = 238 s [4 min]; vinyl sulfonimidamide = 576 s [10 min]; acrylamide = 14091 s [235 min]).
The reactivity is quite high especially when electron withdrawing substituents are introduced onto the sulfonimidamide.
Unfortunately, they did not synthesize any analogues with electron donating groups within the morpholine series.
They did synthesize a couple analogues with a 4-methoxyphenyl group, which attenuated the reactivity towards
GSH only slightly (half-lives: 5c = 196 x [3 min]) and 6d = 842 s [14 min]. Based on these two derivatives,
it seems that substitutions at the imide have a larger effect on reactivity than substitutions on the amine.
I'm only highlighting a few tidbits in this blog post.
The publication has other interesting details and examples characterizing vinyl sulfonimidamides. And it is open access!
Go Check it out!
Generally, it looks like this electrophilic group can be tuned to a broad range of reactivities
depending on the application. As a potential addition to screening libraries, it would be desirable
to reduce the non-specific reactivity with GSH. The chiral center offers an opportunity to synthesize
complementary enantiospecific molecules. I touched on this in an
earlier post, but this approach to
screening offers an immediate control for follow-up experiments and within the screen.
If one enantiomer covalently modifies a protein and the other isomer does not (or has drastically reduced reactivity),
then you can feel pretty confident that an enantiospecific interaction is occurring.
A complementary chiral library could be an application of vinyl sulfonimidamides.
We’ll see if these start popping up in commercial
covalent screening libraries in the near future. It doesn’t look like Enamine has any vinyl
sulfonimidamides in their screening library yet, but maybe soon! I’ll stop there, thanks for reading.
The site does not have a comments section yet! Hopefully, very soon! Until then please drop me a line at jwiden@chemjam.com.
If you provide comments on my articles I reserve the right to post them on this website as additional commentary. My goal is to have an open discussion!